Oxidation Number of Hydrogen
The oxidation state is not always the charge present on the molecule. When H is directly attached to strongly electropositive metals such as K the H atom is present in the form.
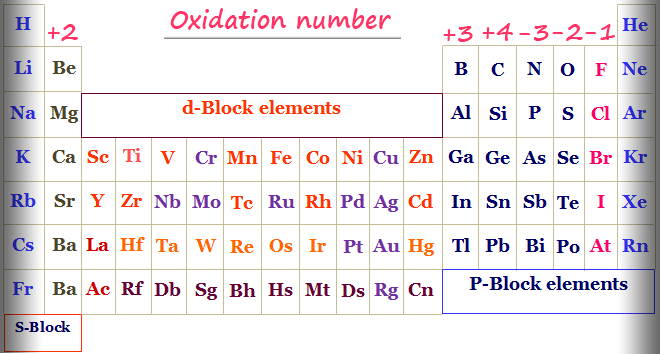
Oxidation Number Periodic Table Elements Definition Rules
Because oxidation number of carbon atom is unknown.
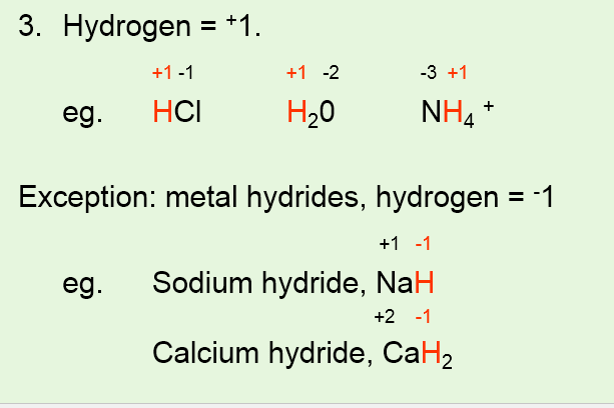
. Oxidation number also referred to as oxidation state is the number that is allocated to elements in a chemical combination. The oxidation number of a monatomic ion equals the. The oxidation number is frequently used interchangeably with an Oxidation state.
For the compound sodium hydride hydrogen is bonded to sodium which is a metal so the oxidation number of hydrogen is -1. Ethanol CH3CH2OH lewis structure should be drawn to find oxidation numbers of elements. To find the correct oxidation number for CaH2 Calcium hydride and each element in the compound we use a few rules and some simple mathFirst since the C.
It is true that the oxidation number of hydrogen is one yet it is a negative number when paired with. Carbon atoms oxidation number is 2Hydrogens oxidation number is 1Oxygens oxidation number is -2. In borane even though B is a non-metal each hydrogen displays an oxidation state of -1 again electronegativity of H is 21 as opposed 20 of B Hope this makes sense.
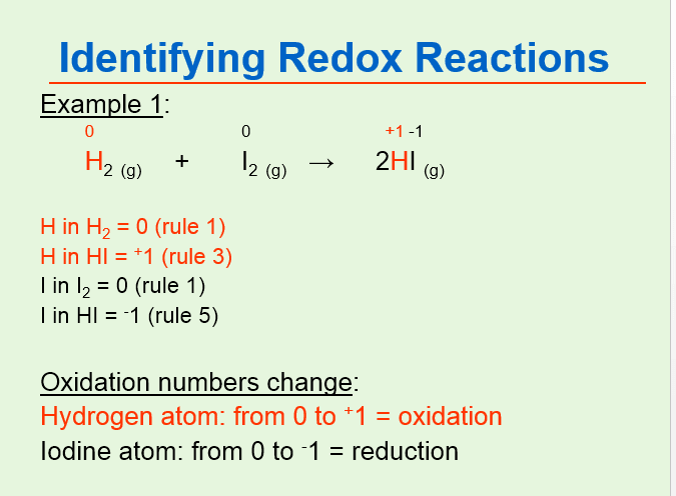
Oxidation number of carbon atom oxidation number of hydrogen oxidation number of oxygen overall charge of HCHO molecule. Because the single oxygen atom has received a total of two electrons one from each hydrogen oxygen has. To find the correct oxidations state of H2 Hydrogen gas and each element in the molecule we use a few rules and some simple mathFirst since the H2 mole.
Oxygen has three possible oxidation. Hydrogen has an oxidation number of 1 in most compounds. Oxidation number of nitrogen atom oxidation number of oxygen atoms oxygen number of hydrogen atom overall charge of HNO 3 molecule.
It is because the stock notation of oxidation numbers is the basis of the periodic property. The oxidation states are used to identify the number of electrons of an atom that has gained or lost. Learn how to calculate or find the oxidation number of elements.
The algebraic sum of the oxidation numbers of. The charge of a monatomic ion is equal to the amount of oxidation electrons in the ion. 6 The oxidation number of hydrogen is 1 when it is combined with more electronegative elements most nonmetals and 1 when it is combined with more electropositive elements.
It can be calculated. The oxidation number of Hydrogen. The major exception is when hydrogen is combined with metals as in N a H ce NaH NaH or L i A l H X 4.
Hydrogen has an oxidation number of 1 when combined with non-metals but it has an oxidation number of -1 when combined with metals. The oxidation number of a free element is always 0. Oxidation number of carbon atom oxidation number of chlorine atoms oxygen number of hydrogen atom overall charge of CHCl 3 molecule.
One carbon atom is at -3 oxidation state and other carbon atom is at -1 oxidation state. Hydrogen has an oxidation number of 1 in water because it has lost one electron. In KH the oxidation number of hydrogen is -1 and the oxidation number of K is 1.
The atoms in He and N 2 for example have oxidation numbers of 0. Home Community Why is the oxidation number of hydrogen in NaBH4 negative one. The oxidation number of hydrogen is 1 except when it is bonded to metals in binary compounds that is compounds containing two elements.
Because oxidation number of nitrogen. Downvote Inorganic chemistry Oxidation Chemistry Metals. Because oxidation number of carbon.

Oxidation Number State Definition Rules How To Find And Examples
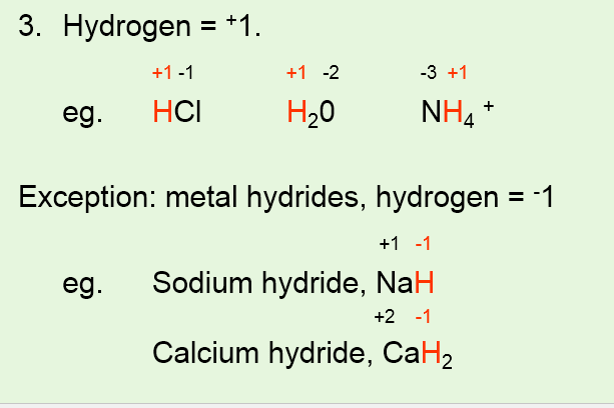
Oxidation Numbers Vce Chemistry
Oxidation Numbers Vce Chemistry
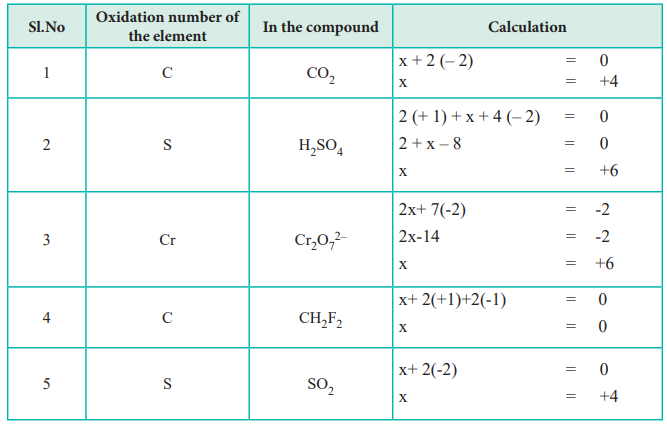
0 Response to "Oxidation Number of Hydrogen"
Post a Comment